
The Sanderson Group: Research on the Chemistry and Biophysics of Lipids
Chemistry Department
Durham University, Durham, DH1 3LE, UK
Research in the Sanderson group focuses on the synthesis and chemical reactivity of lipids and the interactions of
small organic molecules, proteins and peptides with membranes.
Research Areas:
- Probing of the reactivity of drugs and other molecules with membrane lipids and the role of membrane reactivity in disease.
- Understanding the activity of membrane-active peptides and proteins.
- The development of new analytical methods for studying lipids and membranes, including Raman tweezing and linear dichroism spectroscopy.
- The development of model systems for studying the interactions between aromatic amino acids and phospholipids.
- Understanding and optimising the foliar uptake of amino acids by plants.
Selected Publications:
"The Chemical Reactivity of Membrane Lipids",
Genevieve Duché and John M Sanderson, Chem. Rev., 2024,124, in press.
"The association of lipids with amyloid fibrils",
John M. Sanderson, J. Biol. Chem., 2022, 298, 102108.
"Lysis of membrane lipids promoted by small organic molecules:
Reactivity depends on structure but not lipophilicity",
Hannah M. Britt, Aruna S. Prakash, Sanna Appleby, Jackie A. Mosely and John M. Sanderson, Sci. Adv., 2020, 6, eaaz8598.
"Far from Inert: Membrane Lipids Possess Intrinsic Reactivity That Has Consequences for Cell Biology",
John M. Sanderson, BioEssays, 2020, e1900147.
"Lytic Reactions of Drugs with Lipid Membranes",
Hannah M. Britt, Clara A. García-Herrero, Paul W. Denny, Jackie A. Mosely and John M. Sanderson, Chem. Sci., 2019, 10, 674–680.
"The Influence of Cholesterol on Melittin Lipidation in Neutral Membranes",
Hannah M. Britt, Jackie A. Mosely and John M. Sanderson, Phys. Chem. Chem. Phys., 2019, 21, 631–640.
Society Membership:
Member of the British Biophysical Society (committee member),
Biophysical Society
and the Royal Society of Chemistry (FRSC).
News
Recent work from the Sanderson group has focused on the discovery that molecules of all sizes and membrane affinities have the potential to affect the chemistry of membrane lipids. Recent work with small organic molecules have demonstrated a range of activities, from changes in lipid hydrolysis to acyl transfer from the lipid to the molecule. This work has led to the development of the "amyloid lipidation hypothesis", the central tenet of which is that lipidation of amyloid peptides plays a key role in amyloidogenesis.
• A review of the chemical reactivity of membrane lipids
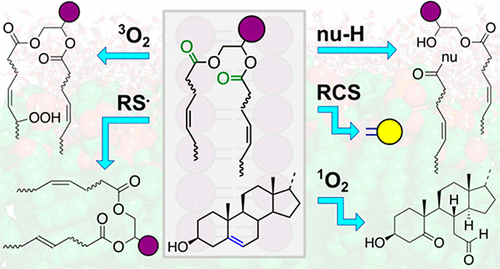 The particular environment of the lipid bilayer, with a water-poor low dielectric core surrounded by a more polar and better hydrated interfacial region,
gives the membrane particular biophysical and physicochemical properties and presents a unique environment for chemical reactions to occur. Many different
types of molecule spanning a range of sizes, from dissolved gases through small organics to proteins, are able to interact with membranes and promote
chemical changes to lipids that subsequently affect the physicochemical properties of the bilayer. This review in Chemical Reviews describes the chemical reactivity
exhibited by lipids in their membrane form, with an emphasis on conditions where the lipids are well hydrated in the form of bilayers.
To read the review, click here.
The particular environment of the lipid bilayer, with a water-poor low dielectric core surrounded by a more polar and better hydrated interfacial region,
gives the membrane particular biophysical and physicochemical properties and presents a unique environment for chemical reactions to occur. Many different
types of molecule spanning a range of sizes, from dissolved gases through small organics to proteins, are able to interact with membranes and promote
chemical changes to lipids that subsequently affect the physicochemical properties of the bilayer. This review in Chemical Reviews describes the chemical reactivity
exhibited by lipids in their membrane form, with an emphasis on conditions where the lipids are well hydrated in the form of bilayers.
To read the review, click here.
• A re-examination of the role of lipids in amyloid fibrils
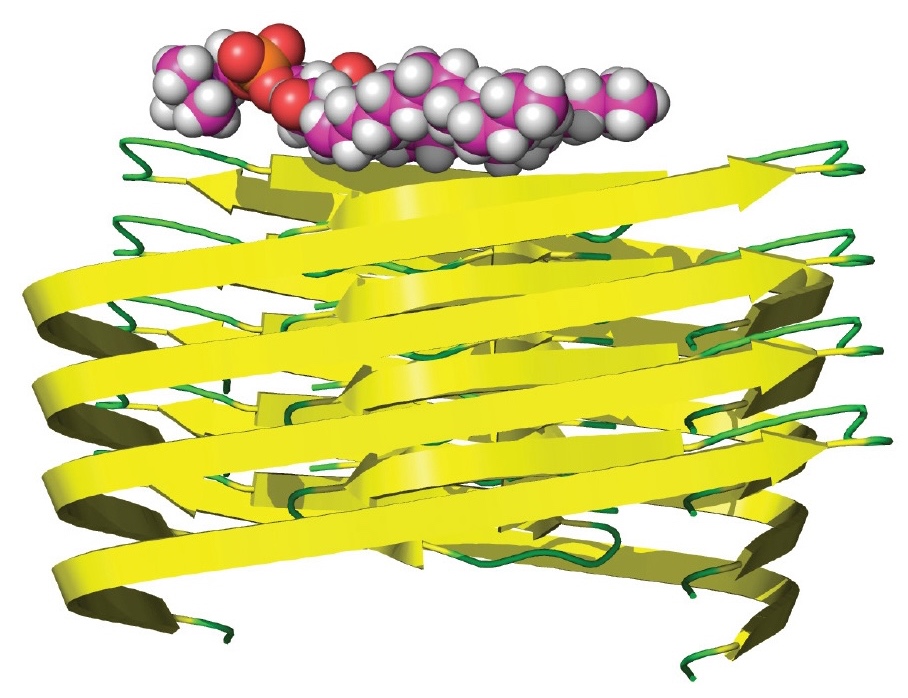 This combined review and opinion piece focuses on evidence for the presence of lipids in amyloid fibrils and the routes by which those lipids may become incorporated.
Chemical analyses of fibril composition, combined with studies to probe the lipid distribution around fibrils, provide evidence that in some cases,
lipids have a strong association with fibrils. In addition, amyloid fibrils formed in the presence of lipids have distinct morphologies and material
properties. It is argued that lipids are an integral part of many amyloid deposits in vivo, where their presence has the potential to influence the
nucleation, morphology, and mechanical properties of fibrils.
To read the article, click here.
This combined review and opinion piece focuses on evidence for the presence of lipids in amyloid fibrils and the routes by which those lipids may become incorporated.
Chemical analyses of fibril composition, combined with studies to probe the lipid distribution around fibrils, provide evidence that in some cases,
lipids have a strong association with fibrils. In addition, amyloid fibrils formed in the presence of lipids have distinct morphologies and material
properties. It is argued that lipids are an integral part of many amyloid deposits in vivo, where their presence has the potential to influence the
nucleation, morphology, and mechanical properties of fibrils.
To read the article, click here.
• Small molecule reactivity with membrane lipids
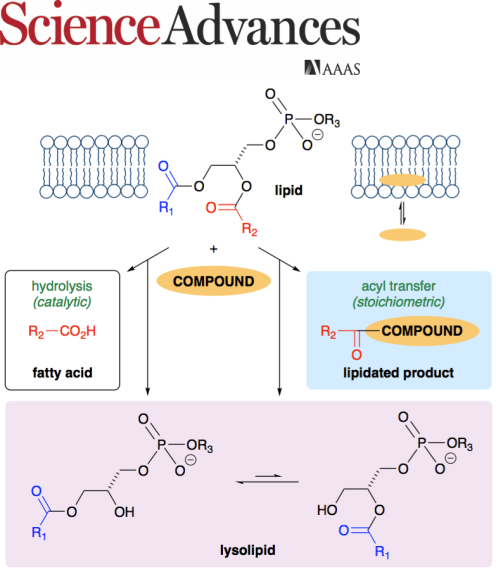 A paper published in Science Advances describes our work to investigate the potential for small organic molecules to influence the
stability of membrane lipids. Several small organic molecules are demonstrated to have significant
membrane-lytic potential despite having a low predicted lipophilicity. In aqueous liposome dispersions,
38 aromatic compounds were tested for their ability to either promote lipid hydrolysis or undergo acyl transfer from the lipid to form a lipidated compound.
The notably higher activity for heterocycles such as amino-substituted benzimidazoles and indazoles demonstrates
the potential to predict or "design-in" lytic activity once the rules that govern reactivity are better understood. The nature of
this chemical instability has significant ramifications for the use or presence of lipids in diverse fields such as materials
chemistry, food chemistry, and cell physiology.
To read the article, click here.
A paper published in Science Advances describes our work to investigate the potential for small organic molecules to influence the
stability of membrane lipids. Several small organic molecules are demonstrated to have significant
membrane-lytic potential despite having a low predicted lipophilicity. In aqueous liposome dispersions,
38 aromatic compounds were tested for their ability to either promote lipid hydrolysis or undergo acyl transfer from the lipid to form a lipidated compound.
The notably higher activity for heterocycles such as amino-substituted benzimidazoles and indazoles demonstrates
the potential to predict or "design-in" lytic activity once the rules that govern reactivity are better understood. The nature of
this chemical instability has significant ramifications for the use or presence of lipids in diverse fields such as materials
chemistry, food chemistry, and cell physiology.
To read the article, click here.
• Has the intrinsic reactivity of membrane lipids been overlooked?
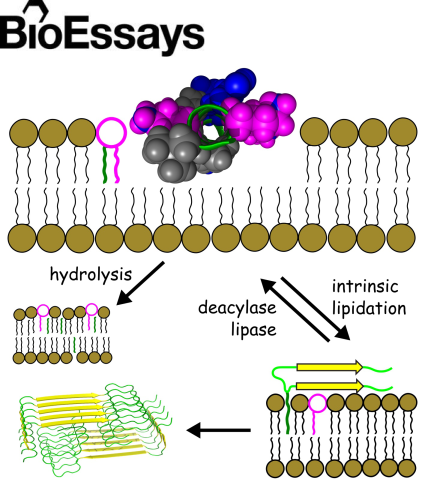 A hypothesis is presented that a fundamental chemical reactivity exists between some non-lipid constituents of cellular membranes and
ester-based lipids, the significance of which is not generally recognised. Many peptides and smaller organic molecules have
now been shown to undergo lipidation reactions in model membranes in circumstances where direct reaction with the lipid is
the only viable route for acyl transfer. Crucially, drugs like propranolol are lipidated in vivo with product profiles that
are comparable to those produced in vitro. Some compounds have also been found to promote lipid hydrolysis. Drugs with high
lytic activity in vivo tend to have higher toxicity in vitro. Deacylases and lipases are proposed as key enzymes that protect
cells against the effects of intrinsic lipidation. The toxic effects of intrinsic lipidation are hypothesized to include a route
by which nucleation can occur during the formation of amyloid fibrils.
To learn more, click here.
A hypothesis is presented that a fundamental chemical reactivity exists between some non-lipid constituents of cellular membranes and
ester-based lipids, the significance of which is not generally recognised. Many peptides and smaller organic molecules have
now been shown to undergo lipidation reactions in model membranes in circumstances where direct reaction with the lipid is
the only viable route for acyl transfer. Crucially, drugs like propranolol are lipidated in vivo with product profiles that
are comparable to those produced in vitro. Some compounds have also been found to promote lipid hydrolysis. Drugs with high
lytic activity in vivo tend to have higher toxicity in vitro. Deacylases and lipases are proposed as key enzymes that protect
cells against the effects of intrinsic lipidation. The toxic effects of intrinsic lipidation are hypothesized to include a route
by which nucleation can occur during the formation of amyloid fibrils.
To learn more, click here.
The Lab
The Sanderson group is located in the Department of Chemistry at Durham University.






