
The Sanderson Group Webpages
Department of Chemistry
Durham University, Durham, UK
Lipid Chemistry and Biophysics
A number of analytical methods are being used in the Sanderson group to examine lipids and membranes. These include spectroscopy (Raman, SAXS), microscopy (confocal, Brewster angle, AFM) and mass spectrometry. In all cases, the aim is to reveal information on chemical processes (e.g. peptide/protein binding) or the chemical constitution of the sample.
Lipidation Reactions
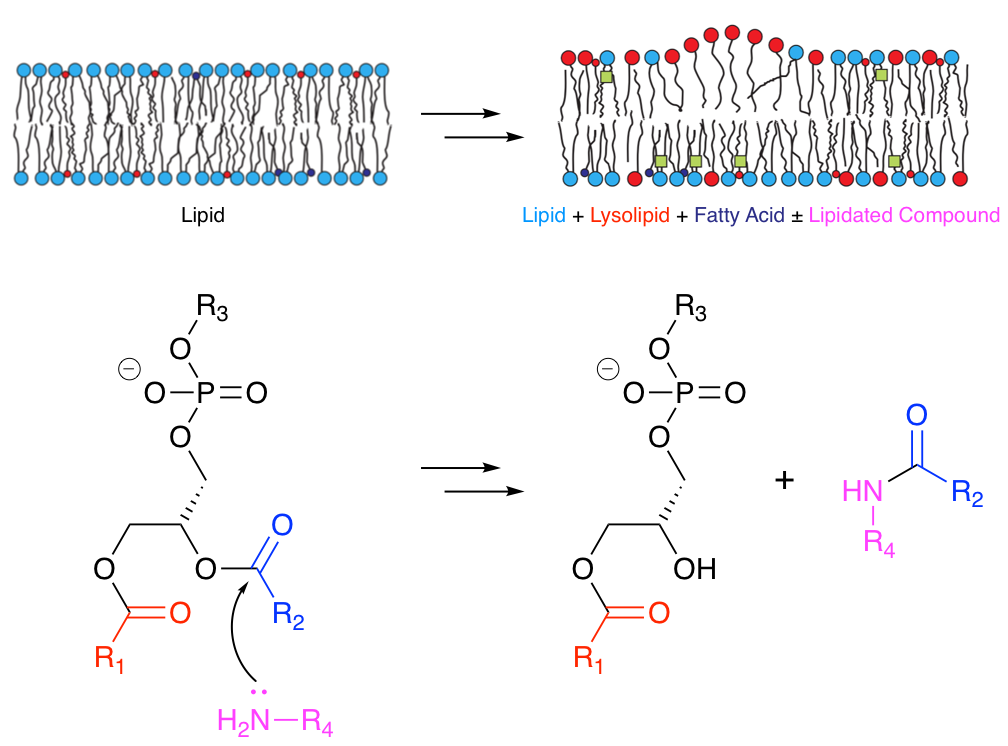
Many molecules that associate with lipid membranes have the potential to undergo direct reactions with the lipid molecules that constitute the bilayer. Depending on the functional groups present, these reactions include transesterification and aminolysis. Aminolysis and transesterification activities were first described for the peptide melittin.1-3 Subsequently we have been able to describe similar reactivity for other peptides.4
More recently we have obtained evidence that acyl transfer can occur from membrane lipids to compounds of lower molecular weight, such as the drug propranolol5 and a number of other small organic molecules.6 This transfer occurs in model liposome systems in vitro and in vivo in Hep G2 cells. In the latter case, incubation of cells with 30 µM propranolol followed by LC-MS analysis led to the identification of 14 different lipidated propranolol species. Other membrane-active drugs did not generate detectable levels of lipidated products, but those that promoted lipid hydrolysis in vitro were found to have lower EC50 values for phospholipidosis. A range of activities has been observed for other organic molecules, including changes in the rate of lipid hydrolysis, both with and without concomitant lipidation. These activities are structure-dependent and do not appear to require high affinity for the membrane.6
In the higher molecular weight regime, we have established that two positions on the lens protein aquaporin-0 (AQP0), are modified with a series of acyl chains, including in decreasing order of abundance: oleoyl, palmitoyl, stearoyl, eicosenoyl, dihomo-γ-linolenoyl, palmitoleoyl and eicosadienoyl.6 The relative abundances of these post-translational modifications (PTMs) mirror the fatty acid composition of lens phosphatidylethanolamine (PE) lipids, as would be expected if they arise by a process of 'intrinsic lipidation', involving membrane lipids as the acyl donor. PE is the major lipid class in the membrane leaflet proximal to the sites of modification (the N-terminus and side chain of Lys238). The levels of modification (typically < 50% lipidation at each site) and lack of any known enzyme consensus sequence at these sites further support intrinsic lipidation as the method by which these PTMs arise.
The research described above has led to a hypothesis that intrinsic lipidation reactions are more prevalent in biological systems than generally recognised and may underpin some key molecular processes associated with disease, such as the nucleation of amyloid fibrils.7,8 Deacylases such as sirtuins may play a vital role in reversing intrinsic lipidation reactions in vivo.8
Mass Spectrometry
In collaboration with Jackie Mosely (York), we are developing analytical methods for the complete analysis of lipids. Complete analysis of glycerophospholipids requires identification of the headgroup and both acyl chains, including the number, position and geometry of double bonds. In early experiments we compared different methods for analysing lipids by MALDI-MSMS, including the effects of the matrix and additives to the matrix mixture. From this, specific pairs of related ions that could be used to distinguish the position of acyl chains on the glycerol backbone were identified, although it became clear that careful calibration is required for all experiments of this kind, as the intensities of key fragments are instrument-specific.11 More recently, we have developed analytical methods for the analysis of peptide-lipid samples, using liquid chromatography combined with tandem MS. This work has been fundamental in identifying the lipidation processes described above that occur through transfer of acyl groups from lipids to drugs, peptides, and proteins.1-6 A key defining feature of our laboratory is the ability to chemically synthesise labelled lipids and sterols to assist with characterisation.10
Spectroscopy
Linear dichroism (LD) spectroscopy, using liposomal membranes aligned by shear flow, has proved to be a useful tool for monitoring the kinetics of peptide binding to membranes.12-14 For the analysis of lipid membrane composition, Raman spectroscopy of single liposomes held in optical tweezers was demonstrated by us to be a useful analytical tool (in collaboration with Dr A D Ward).15
Microscopy
We have combined thin-film methodologies such as Brewster angle microscopy (BAM, with Prof A Beeby, Durham) and AFM of protein films deposited onto ODS-treated silicon. This has enabled us to examine the membrane properties of viral matrix proteins.16 In collaboration with Ritu Kataky (Durham), scanning electrochemical microscopy (SECM) has been deployed to study the channel gating properties of a gramicidin analogue modified with nicotinamide.17
References
- "The Influence of Cholesterol on Melittin Lipidation in Neutral Membranes", Hannah M. Britt, Jackie A. Mosely and John M. Sanderson, Phys. Chem. Chem. Phys., 2019, 21, 631–640. (green open access version)
- "The Innate Reactivity of a Membrane Associated Peptide Towards Lipids: Acyl Transfer to Melittin Without Enzyme Catalysis", Robert H. Dods, Jackie A. Mosely and John M. Sanderson, Org. Biomol. Chem., 2012, 10, 5371–5378. (green open access version)
- "Acyl Transfer from Phosphocholine Lipids to Melittin", Catherine J. Pridmore, Jackie A. Mosely, Alison Rodger and John M. Sanderson, Chem. Commun., 2011, 47, 1422-1424.
- "Acyl Transfer from Membrane Lipids to Peptides is a Generic Process", Robert H. Dods, Burkhard Bechinger, Jackie A. Mosely and John M. Sanderson, J. Mol. Biol., 2013, 425, 4379–4387. (green open access version)
- "Lytic Reactions of Drugs with Lipid Membranes", Hannah M. Britt, Clara A. García-Herrero, Paul W. Denny, Jackie A. Mosely and John M. Sanderson, Chem. Sci., 2019, 10, 674–680. (gold open access version)
- "Lysis of membrane lipids promoted by small organic molecules: Reactivity depends on structure but not lipophilicity", Hannah M. Britt, Aruna S. Prakash, Sanna Appleby, Jackie A. Mosely and John M. Sanderson, Sci. Adv., 2020, 6, eaaz8598. (gold open access version)
- "Far from Inert: Membrane Lipids Possess Intrinsic Reactivity That Has Consequences for Cell Biology", John M. Sanderson, BioEssays, 2020, e1900147. (green open access version)
- "The association of lipids with amyloid fibrils", John M. Sanderson, J. Biol. Chem., 2022, 298, 102108. (gold open access version)
- "The lipidation profile of aquaporin-0 correlates with the acyl composition of phosphoethanolamine lipids in lens membranes", Vian S. Ismail, Jackie A. Mosely, Antal Tapodi, Roy A. Quinlan and John M. Sanderson, Biochim. Biophys. Acta - Biomembr., 2016, 1858, 2763–2768. (green open access version)
- "Optimised conditions for the synthesis of 17O and 18O labelled cholesterol", Celia de la Calle Arregui, Jonathan A. Purdie, Catherine A. Haslam, Robert V. Law and John M. Sanderson, Chem. Phys. Lipids, 2016, 195, 58–62.
- "The Reproducibility of Phospholipid Analyses by MALDI-MSMS", Catherine J. Pridmore, Jackie A. Mosely and John M. Sanderson, Analyst, 2011, 136, 2598–2605.
- "The Association of Defensin HNP-2 with Negatively Charged Membranes: A Combined Fluorescence and Linear Dichroism Study", Catherine J. Pridmore, Alison Rodger and John M. Sanderson, Biochim. Biophys. Acta - Biomembr., 2016, 1858, 892–903. (gold open access version)
- "Resolving the Kinetics of Lipid, Protein and Peptide Diffusion in Membranes", John M. Sanderson, Mol. Membr. Biol., 2012, 29, 118–143. (green open access version, local version)
- "Peptide Adsorption to Lipid Bilayers: Slow Processes Revealed by Linear Dichroism Spectroscopy", Sue M. Ennaceur, Matthew R. Hicks, Catherine J. Pridmore, Tim R. Dafforn, Alison Rodger and John M. Sanderson, Biophys. J., 2009, 96, 1399–1407.
- "Analysis
of Liposomal Membrane Composition Using Raman Tweezers",
John M. Sanderson and Andrew D. Ward, Chem. Commun., 2004, 1120.
- "Influence of Lipids on the Interfacial Disposition of Respiratory Syncytial Virus Matrix Protein", Helen K. McPhee, Jennifer L. Carlisle, Andrew Beeby, Victoria A. Money, Scott M. D. Watson, Robert P. Yeo and John M. Sanderson, Langmuir, 2011, 27, 304-311.
- "A Gramicidin Analogue that Exhibits Redox Potential-Dependent Cation Influx", Thomas J. Jackson, John M. Sanderson, and Ritu Kataky, Sensors and Actuators B, 2008, 130, 630-637.