
The Sanderson Group Webpages
Department of Chemistry
Durham University, Durham, UK
Applications of Laser Tweezing to the Study of Liposomal Membranes
In collaboration
with Dr Andrew Ward, we
are seeking to apply optical
entrapment (laser tweezing) methodologies to the study of liposomal
membranes. Laser tweezing allows us to observe
a single liposome or pair of interacting liposomes for a prolonged
period of time, and therefore observe slow membrane phenomena such as lipid exchange.
In initial work we used liposomes whose contents or membranes were
labelled with fluorescent markers, and were able to hold trapped
liposomes for periods in excess of 1 h. Some problems arising from
photobleaching were encountered however. In more recent work, we have
turned to Raman spectroscopy in order to study membrane structure.
Raman spectroscopy offers the large advantage that no
labelling is required in order to obtain spectra. We have demonstrated
that it is feasible to
obtain Raman spectra from individual trapped liposomes (Figure 1), and
then use these for analytical purposes in order to examine membrane
composition.1

Figure 1
Raman spectra obtained from
single trapped liposomes (1 micron diameter) composed of pure DMPC (A) or DOPC
(B) in phosphate buffered saline at
pH7.2.
Key signals in the DMPC spectrum are at 1060, 1085 and 1125 cm-1
(C-C skeletal stretching vibrations), 1296 cm-1
(CH2 twist) and 1445 cm-1
(CH2 bend). In the DOPC spectrum, additional
signals of note are found at 1264 cm-1 and
1655 cm-1 (cis C=C stretching vibration).
By using the spectra above as reference spectra, we are able to analyse
the composition of membranes. An example is shown below for the
partitioning of hexafluoroisopropanol (HFIP) into DMPC membranes
(Figure 2). From the data it is apparent that HFIP interacts favourably
with DMPC membranes, considering the signal strength from trapped
liposomes in the presence of low HFIP concentrations.
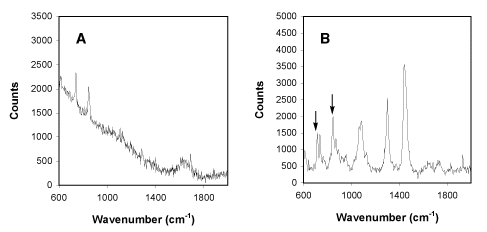
Figure 2
Raman spectra obtained from a 2.5% solution of hexafluoroisopropanol (HFIP) in phosphate buffered
saline (A) and from single
trapped liposomes (1 micron diameter) composed of pure DMPC (B) in phosphate buffered saline at
pH7.2 in the presence of 0.25% hexafluoroisopropanol (HFIP).2
Key signals in the HFIP spectrum are at 740 and 846 cm-1.
Signals from HFIP in the HFIP/DMPC mixture are indicated by arrows.
High-Resolution Microscopy of Peptides Binding to Membranes
In collaboration
with Professor
Ritu Kataky
(University of Durham), we
are seeking to obtain high resolution images of synthetic peptides
binding to the surface of lipid membranes and assembling into
transmembrane channels. In particular, we are interested in
characterising
the assembly process and identifying the specific lipid molecules
that preferentially associate with the assembling channel. Our
microscopic techniques, Scanning Electrochemical Microscopy (SECM)
and the Scanning Kelvin Probe Microscopy (SKP), are well suited
to this end. By inserting unusual amino acids at particular sites
in a peptide template, and determining the subsequent effects
on the assembly process, we hope to establish structure-function
relationships for peptide insertion into lipid bilayers.
- "Analysis of Liposomal Membrane Composition Using Raman Tweezers", John M. Sanderson and Andrew D. Ward, Chem. Commun., 2004, 1120.
- "The Formation of Micellar Aggregates Following the Addition of Hexafluoroisopropanol to Phospholipid Membranes", Sue M. Ennaceur and John M. Sanderson, Langmuir, 2005, 21, 552-561.