
The Sanderson Group Webpages
Department of Chemistry
Durham University, Durham, UK
Intrinsic Lipidation
During the course of kinetics studies on the association of the peptide melittin with unilamellar membranes, it became apparent that the chemical identity of the species present in the samples was continually changing.2 Initial MALDI-MS studies identified additional species in the sample corresponding to adducts of melittin with lipid acyl groups, alongside corresponding lyso-PCs. These had formed by reaction of amino groups on the peptide with the glyceryl esters of the lipid in an amidation reaction (Figure 1). Intrinsic lipidation reaction occurs without enzyme catalysis and will potentially occur with any membrane-embedded molecule.
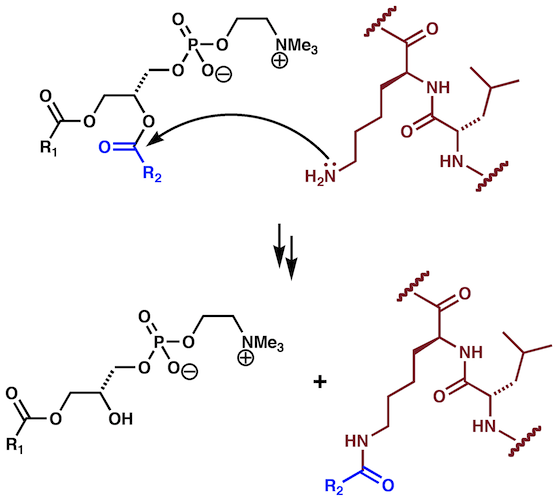
Figure 1 Reaction Scheme for the Intrinsic Lipidation of Peptides
Through the use of tandem mass spectrometry, including LC-MS, LC-MS2 and LC-MS3 we have established that acyl transfer occurs from both the sn-1 and sn-2 positions of PCs with almost equal probability.2,3 The sensitivity of these analytical methods is such that 5 pmol of lipidated peptide can be detected with a mass accuracy of 1–3 parts per million at peptide:lipid ratios of 1:100. Lipidated peptides could be detected in melittin/POPC samples after periods as short as 4 h. The lipidation reaction exhibits some chemoselectivity, with acyl transfer from PG observed in DOPC/DMPG mixtures, but not from PS in DOPC/DPPS mixtures.2
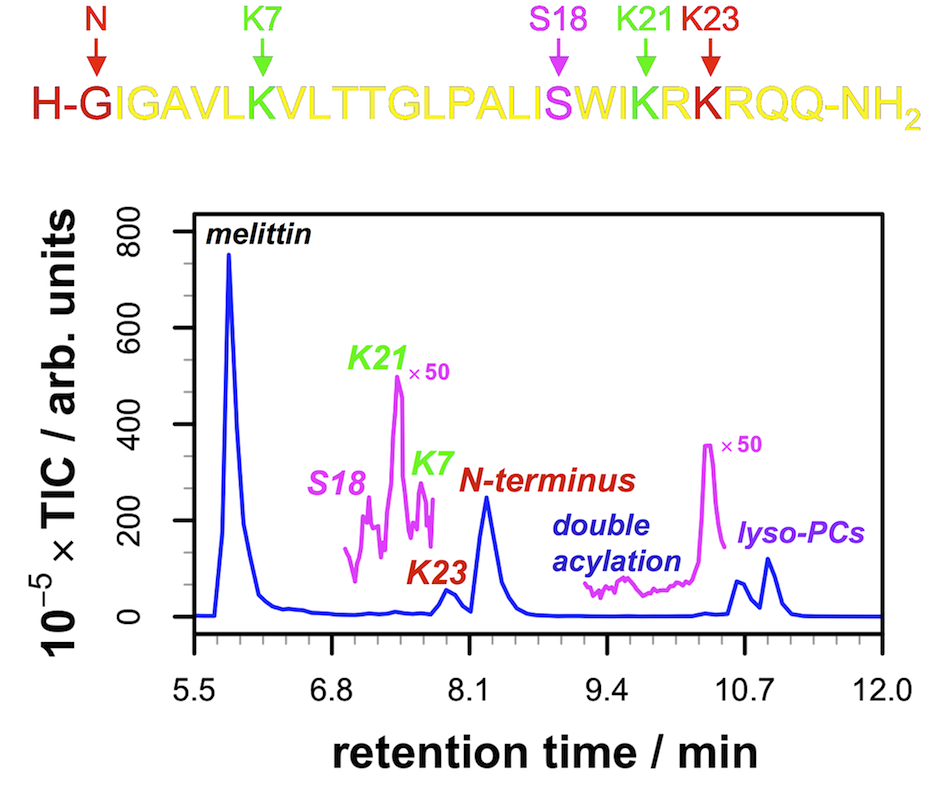
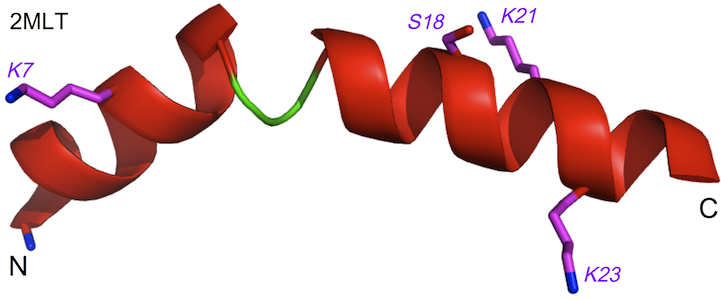
Figure 2 LC-MS trace (left) from a sample of melittin incubated with unilamellar vesicles of POPC for 48 h, indicating the identities of the major and minor peaks in the trace, as well as the location of these sites on the structure of melittin (right, from PDB entry 2MLT).
The products of double lipidation of the peptide could also be detected, as well as putative products arising from a transesterification reaction involving Ser18 of melittin (Figure 2). Other peptides, including Magainin II and PGLa, also undergo intrinsic lipidation.1 Cholesterol inclusion in the membrane has been shown to have a significant effect on melittin lipidation rates.4 Peptide lipidation can also occur from lysolipids and can direct the folding of unstructured peptides.5 The effects of cholesterol on lipidation and the ability to influence peptide structure have led to hypotheses that intrinsic lipidation may have a role in amyloid formation.6,7
More recently, intrinsic lipidation of low molecular weight organic molecules has been described.8,9 Intrinsic lipidation of proteins is also a distinct possibility.10,11
References
- "Acyl Transfer from Membrane Lipids to Peptides is a Generic Process", Robert H. Dods, Burkhard Bechinger, Jackie A. Mosely and John M. Sanderson, J. Mol. Biol., 2013, 425, 4379–4387.
- "The Innate Reactivity of a Membrane Associated Peptide Towards Lipids: Acyl Transfer to Melittin Without Enzyme Catalysis", Robert H. Dods, Jackie A. Mosely and John M. Sanderson, Org. Biomol. Chem., 2012, 10, 5371–5378. in press.
- "Acyl Transfer from Phosphocholine Lipids to Melittin", Catherine J. Pridmore, Jackie A. Mosely, Alison Rodger and John M. Sanderson, Chem. Commun., 2011, 47, 1422–1424.
- "The Influence of Cholesterol on Melittin Lipidation in Neutral Membranes", Hannah M. Britt, Jackie A. Mosely and John M. Sanderson, Phys. Chem. Chem. Phys., 2019, 21, 631–640. (green open access version)
- "Peptide lipidation in lysophospholipid micelles and lysophospholipid-enriched membranes", Vian S. Ismail, Hannah M. Britt, Jackie A. Mosely and John M. Sanderson, Faraday Discuss., 2021, 232, 282–294. (gold open access version)
- "The association of lipids with amyloid fibrils", John M. Sanderson, J. Biol. Chem., 2022, 298, 102108. (gold open access version)
- "The chemical reactivity of membrane lipids", Genevieve Duché and John M. Sanderson, Chem. Rev., 2024, 124, in press. (gold open access version)
- "Lysis of membrane lipids promoted by small organic molecules: Reactivity depends on structure but not lipophilicity", Hannah M. Britt, Aruna S. Prakash, Sanna Appleby, Jackie A. Mosely and John M. Sanderson, Sci. Adv., 2020, 6, eaaz8598. (gold open access version)
- "Lytic Reactions of Drugs with Lipid Membranes", Hannah M. Britt, Clara A. García-Herrero, Paul W. Denny, Jackie A. Mosely and John M. Sanderson, Chem. Sci., 2019, 10, 674–680. (gold open access version)
- "Far from Inert: Membrane Lipids Possess Intrinsic Reactivity That Has Consequences for Cell Biology", John M. Sanderson, BioEssays, 2020, e1900147. (green open access version)
- "The lipidation profile of aquaporin-0 correlates with the acyl composition of phosphoethanolamine lipids in lens membranes", Vian S. Ismail, Jackie A. Mosely, Antal Tapodi, Roy A. Quinlan and John M. Sanderson, Biochim. Biophys. Acta - Biomembr., 2016, 1858, 2763–2768. (green open access version)