
The Sanderson Group Webpages
Department of Chemistry
Durham University, Durham, UK
Linear Dichroism Spectroscopy
In collaboration with Professor Alison Rodger, we have used linear dichroism (LD) spectroscopy as a tool for studying the association of peptides with membranes. LD is the differential absorption of left- and right-handed linear polarised light. To observe an LD signal, chromophores (and by inference their parent molecules) need to be aligned with respect to the experimental frame of reference. To achieve this with lipid membranes, unilamellar liposomes are placed in a couette cell, in which the liposomes become aligned by shear flow (Figure 1).

Figure 1 (a) Schematic of a couette cell apparatus to produce shear-aligned liposomes; (b) a model of a shear-deformed liposome as a cylinder.
This then permits the observation of LD from any species that become oriented by binding to the membrane. In the case of peptides, the absence of an LD signal from free (non-membrane associated) peptide offers particular experimental advantages. Another key advantage of LD, when compared with CD, is a higher sensitivity, enabling experiments to be conducted at low peptide:lipid ratios under pseudo first-order conditions. Finally, the LD spectra of the bound peptide states provide information on the alignment of the peptide with the experimental frame of reference, and by inference the membrane. When applied to model peptides, we have been able to discern at least two, and sometimes three, stages in binding to membranes.1,2 These can be successfully modelled as consecutive first order processes. The first process is rapid (seconds timescale), followed by one or more slower phases (minutes to hours). The associated LD spectra are indicative of a loosely bound peripheral state and a transmembrane orientation (Figure 2).
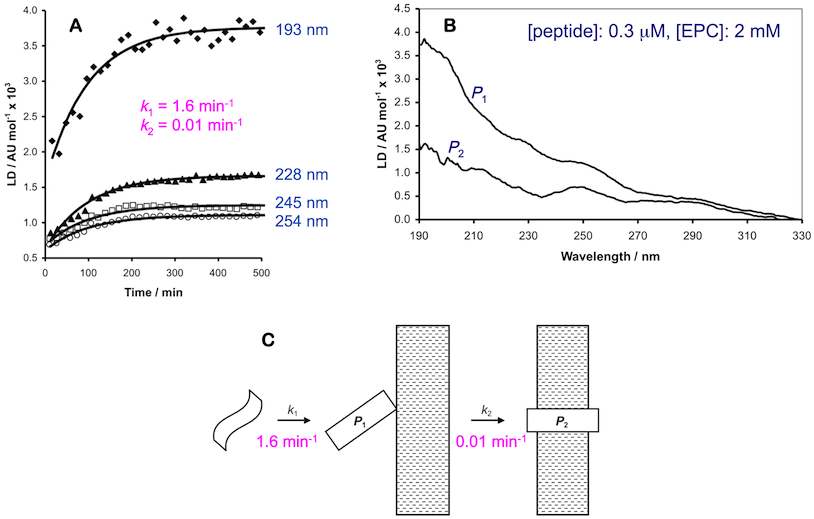
Figure 2 (a) Examples of LD data at multiple wavelengths obtained from peptides binding to liposomal membranes (points=experimental values, lines=curves obtained by modelling the raw data using integrated rate equations); (b) example of calculated LD spectra for two bound states; (c) schematic showing how the LD data can be interpreted.
In these and subsequent experiments with melittin,3 equilibration of the system required >1 hour. The fast initial phase occurs on a similar time frame to marker release and may be more relevant for peptide toxicity (i.e. antimicrobial activity). During the longer phase, in control experiments using synthetic melittin, acyl transfer from the lipid to the peptide was observed, which is documented elsewhere.4,5 From these experiments it is apparent that some peptide-lipid systems do not reach equilibrium on an experimental timescale.
References
- "The Association of Defensin HNP-2 with Negatively Charged Membranes: A Combined Fluorescence and Linear Dichroism Study", Catherine J. Pridmore, Alison Rodger and John M. Sanderson, Biochim. Biophys. Acta - Biomembr., 2016, 1858, 892–903.
- "Peptide Adsorption to Lipid Bilayers: Slow Processes Revealed by Linear Dichroism Spectroscopy", Sue M. Ennaceur, Matthew R. Hicks, Catherine J. Pridmore, Tim R. Dafforn, Alison Rodger and John M. Sanderson, Biophys. J., 2009, 96, 1399-1407.
- "The Synergistic Action of Melittin and Phospholipase A2 with Lipid Membranes: Development of Linear Dichroism for Membrane-Insertion Kinetics", Angeliki Damianoglou, Alison Rodger, Catherine J. Pridmore, Timothy R. Dafforn, Jackie A. Mosely, John M. Sanderson and Matthew R. Hicks, Protein Pept. Lett., 2010, 17, 1351-1362.
- "The Innate Reactivity of a Membrane Associated Peptide Towards Lipids: Acyl Transfer to Melittin Without Enzyme Catalysis", Robert H. Dods, Jackie A. Mosely and John M. Sanderson, Org. Biomol. Chem., 2012, 10, in press.
- "Acyl Transfer from Phosphocholine Lipids to Melittin", Catherine J. Pridmore, Jackie A. Mosely, Alison Rodger and John M. Sanderson, Chem. Commun., 2011, 47, 1422-1424.