
The Sanderson Group Webpages
Department of Chemistry
Durham University, Durham, UK
The Structure of the Matrix (M) Protein from Respiratory Syncytial Virus (RSV)

Respiratory Syncytial Virus (RSV) is an enveloped virus from the order Mononegavirales, which also includes Ebola, Marburg virus, Newcastle disease virus, measles and rabies viruses. In common with other enveloped viruses, which have an outer membrane, a small number of RSV proteins are directly involved in interactions with the membrane. These proteins, including the matrix (M) protein, play key roles in the structure of the virion and the processes involved in virion maturation, including budding from the host cell. In collaboration with Dr Victoria Money and Dr Paul Yeo, we solved the crystal structure of the full-length M protein from RSV at a resolution of 1.6 Å.1 The structure reveals interesting structural details that indicate the likely sites of membrane interaction of the protein and the means by which the protein can control virion morphology. The structure is defined by two domains of approximately equal size that are separated by an unstructured linker region. An extensive surface of positive electrostatic density extends across both domains and the linker.
Characterisation of the Membrane Interactions of RSV M
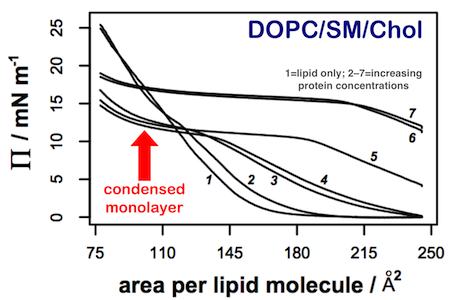
We are characterising the innate membrane binding properties of the RSV M protein using a combination of thin film tensiometry (combined with Brewster angle microscopy (BAM) and AFM of deposited films) and microscopy experiments on model lipid bilayers.2 Thin film experiments have demonstrated that the protein penetrates most lipid films at the air-water interface, forming mixed surface layers that retain the protein beyond its equilibrium spreading pressure in the absence of lipid. The only lipid film to exhibit any significant difference in behaviour was a 1:1:1 mixture of DOPC/Cholesterol/Sphingomyelin. This lipid mixture exhibited microphase separation in both the presence and absence of protein (as demonstrated by BAM). AFM on deposited protein films indicated differences in binding between liquid-ordered and liquid-disordered phases.
References
- "Surface Features of a Mononegavirales Matrix Protein Indicate Sites of Membrane Interaction", Victoria A. Money, Helen K. McPhee, Jackie A. Mosely, John M. Sanderson and Robert P. Yeo, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 4441-4446.
- "Influence of Lipids on the Interfacial Disposition of Respiratory Syncytial Virus Matrix Protein", Helen K. McPhee, Jennifer L. Carlisle, Andrew Beeby, Victoria A. Money, Scott M. D. Watson, Robert P. Yeo and John M. Sanderson, Langmuir, 2011, 27, 304-311.